
to Chem Number of lone pair present at
It is helpful if you: Try to draw the I 3- Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to I 3- for more practice. List of Lewis Structures Lewis Structures for I3-. Step-by-step tutorial for drawing the Lewis Structure for I3-.

SOLVED . Draw the Lewis structure of the following organic compounds
The triiodide ion (I3-) is an anion that is formed by combining three iodine atoms. It is a common reagent in chemistry, often used in redox reactions and as an indicator for starch in iodometry. The ion has a linear shape, with the three iodine atoms arranged in a straight line. Drawing the Lewis Structure of I3-

Gallery For > I3 Lewis Structure Molecular geometry, Vsepr theory
Triiodide in Chemistry usually refers to the Triiodide ion, I3- This anion, one of the polyhalogen ions, is composed of 3 iodine atoms and is formed by combining the aqueous solution of iodine and iodide salts. A few salts of the anion have been isolated, including ammonium Triiodide ( [NH4]+[I3]− and thallium (I) Triiodide (Tl+ [I3]−)).

Charges formelles sur les ions polyatomiques
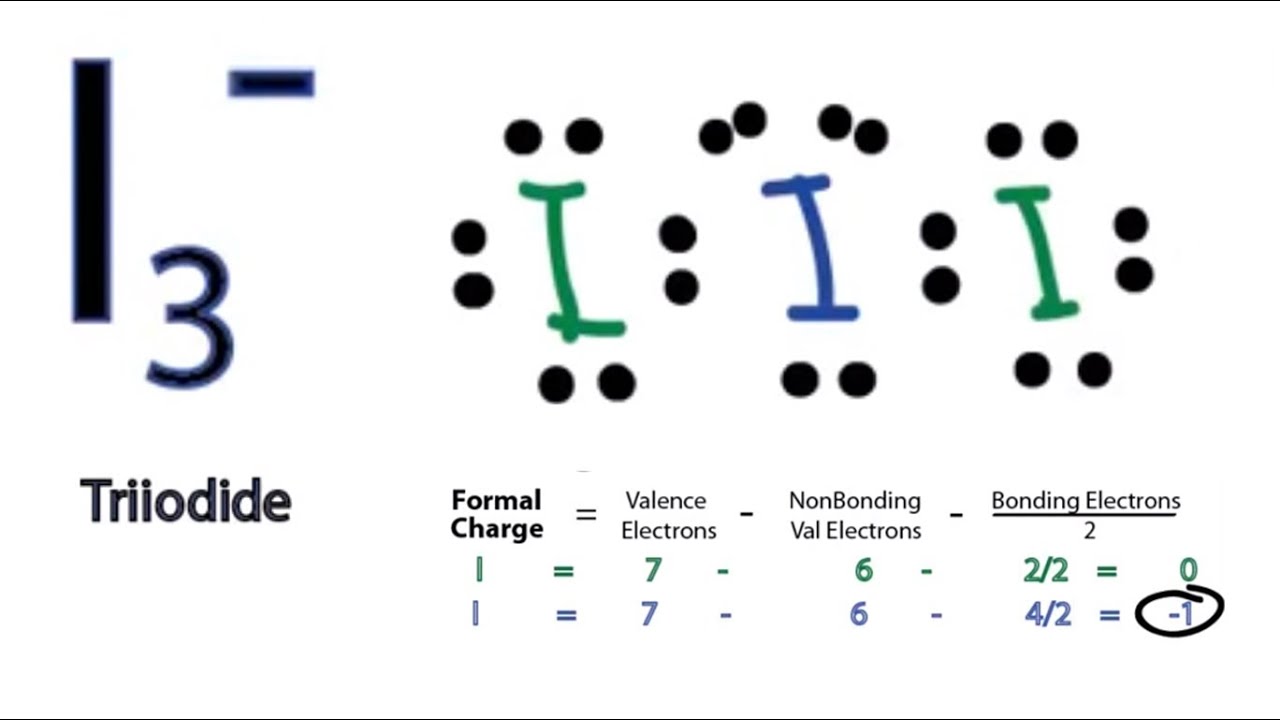
I3- (triiodide) lewis structure has three Iodine atoms (I). There are 2 single bonds between each Iodine atom (I). There are 3 lone pairs on all the three Iodine atom. There is a -1 formal charge on the central Iodine atom (I).

Number of Lone Pairs and Bonding Pairs for I3 YouTube
Hello Guys,We are back with one of the most requested videos on Geometry of Molecules- I3- Lewis structure. It is a chemical formula for the Triiodide ion. T.

I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO
In the I 3- Lewis structure, there are two single bonds around the iodine atom, with two other iodine atoms attached to it, and on each iodine atom, there are three lone pairs. Also, there is a negative (-1) charge on the center iodine atom. I3- Lewis Structure - How to Draw the Lewis Structure for I3- Watch on Contents Steps

How to Draw the Lewis Dot Structure for I3 Triiodide ion YouTube
Contents show Lewis Structure of I3 Anyone wanting to know in-depth about a molecule needs to learn about the Lewis Structure. Why is it so necessary to have an idea on this topic? Well, Lewis Structure is the name given to the diagrammatic representation of chemical bonding occurring between atoms inside a molecule.
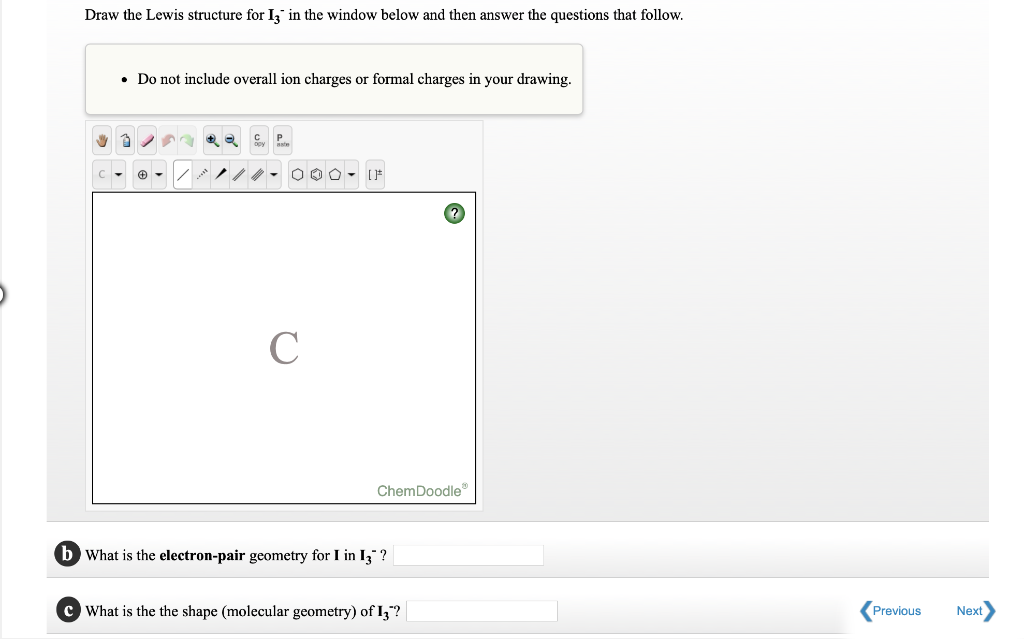
Solved Draw the Lewis structure for I3 in the window below
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
I3 Lewis Structure How to Draw the Lewis Structure for I3 YouTube
3. -. ) - Lewis Structure. In the lewis structure of triiodide ion (I 3- ), there are two I-I bonds. and one iodine atom is located as the center atom. Each iodine atom has 3 lone pairs and center iodine atom have -1 charge. We will learn how to draw the lewis structure of I 3- step by step in this tutorial.
I3 Lewis Structure How To Discuss
I3- Lewis structure or lewis dot structure is a simple representation of the electronic structure of a molecule that briefs about the number of bonds formed, number of bond pairs required to fulfill the octet rule and lone pairs available.
Draw the Lewis dot structure of I3 Take a picture of … SolvedLib
Lewis structure of I3- ion (triiodide) contains two single bonds between each Iodine (I) atom. All the three Iodine atoms have three lone pairs on it, and the central iodine atom has -1 formal charge. Let's draw and understand this lewis dot structure step by step.

I3 Lewis Structure Triiodide Ion YouTube
The Lewis structure for the Triiodide Ion (I3-) consists of three iodine atoms bonded together with a single bond and two lone pairs on each iodine atom. How do you determine the formal charge of each atom in the Triiodide Ion?
[Solved] 1.Which of the options below ranks the following bonds from
This chemistry video tutorial explains how to draw the lewis structure of I3-. It also discusses the molecular geometry, bond angle, hybridization, and formal charges of the triiodide ion..
Get I3 Lewis Structure Molecular Geometry most complete GM
I3- Lewis Structure, Shape, Hybridization and Polarity Written by Priyanka in Lewis Structure It is important to know the Lewis structure of a molecule to understand its physical properties, hybridization, and shape of the molecule.

Lewis Dot Structure of I3 (Triiodide Ion) YouTube
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
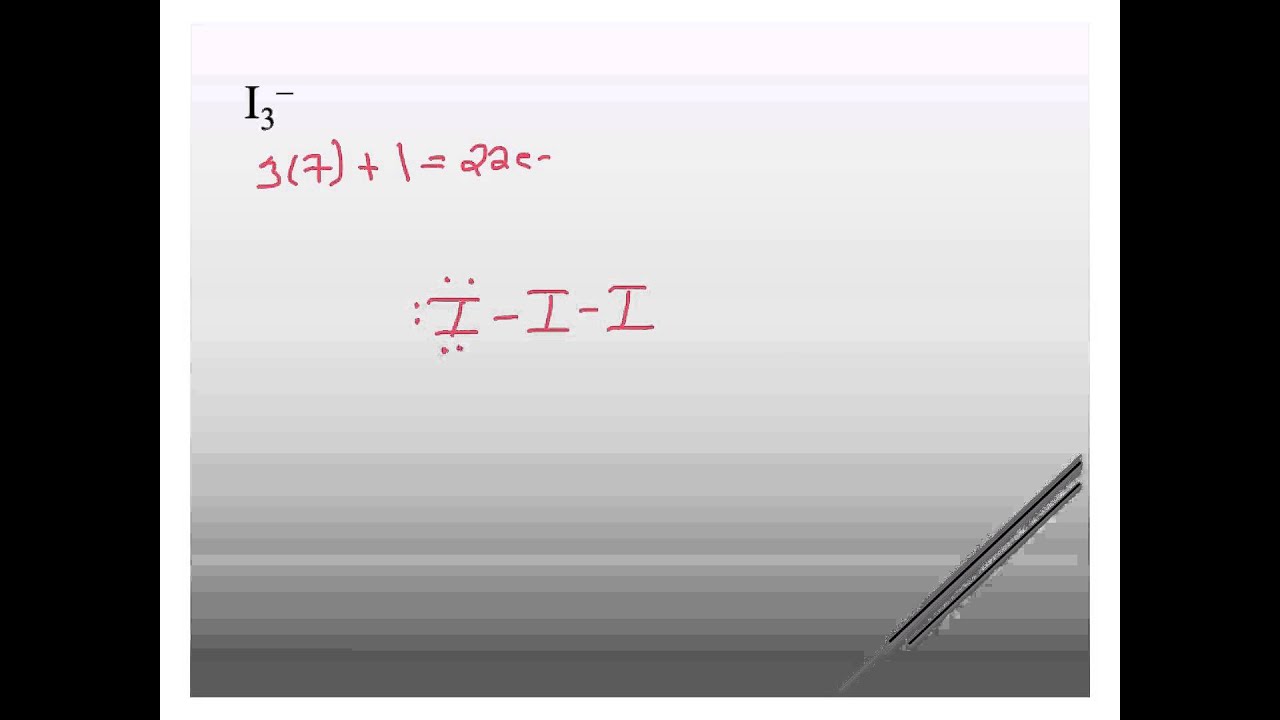
I3 Lewis structure YouTube
Drawing the Lewis Structure for I 3-Viewing Notes: I 3-has a negative charge (and is therefore a negative ion or anion). That means that it has an extra electron that needs to be taken into account.. Let's do the I3- Lewis structure. On the periodic table, Iodine is in group 7 or 17. It has seven valence electrons. But we have three of them.